瀬尾 珠恵
Tamae Seo
D3
発表論文
-
12.
Mechanochemistry-Directed Ligand Design: Development of a High-Performance Phosphine Ligand for Palladium-Catalyzed Mechanochemical Organoboron Cross-Coupling
Seo, T.; Kubota, K.*; Ito, H.* J. Am. Chem. Soc. 2023, Just Accepted
DOI: 10.1021/jacs.2c13543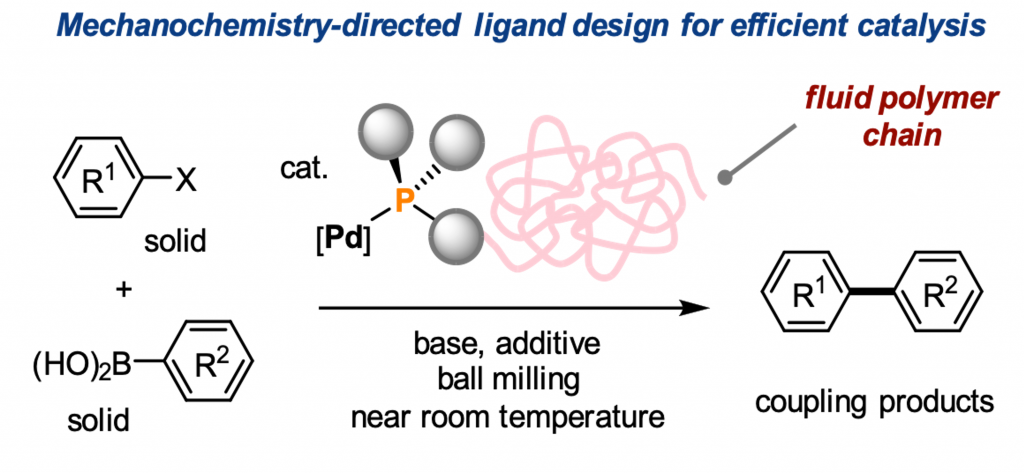
-
11.
Solid-state cross-coupling reactions of insoluble aryl halides under polymer-assisted grinding conditions
Kubota, K.*; Seo, T.; Ito, H.* Faraday Discuss. 2023, 241, 104–113.
DOI: 10.1039/D2FD00121G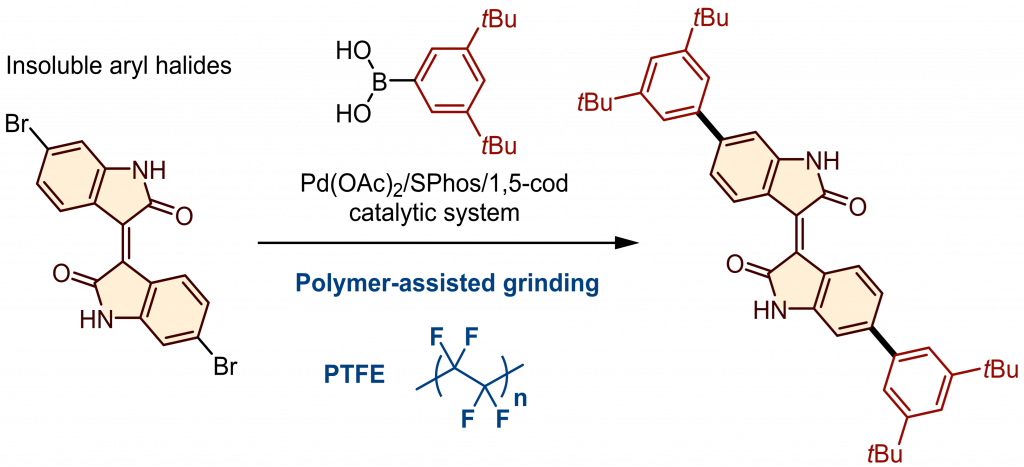
-
10.
Palladium-catalyzed solid-state borylation of aryl halides using mechanochemistry
Kubota, K.*; Baba, E.; Seo, T.; Ishiyama, T.; Ito, H.* Beilstein J. Org. Chem. 2022, 18, 855.
DOI: 10.3762/bjoc.18.86
-
9.
Insight into the Reactivity Profile of Solid-State Aryl Bromides in Suzuki-Miyaura Cross-Coupling Reactions Using Ball Milling
Kubota, K.*; Kondo, K.; Seo, T.; Ito, H.* Synlett 2022, 33, 898–902.
DOI: 10.1055/a-1748-3797 -
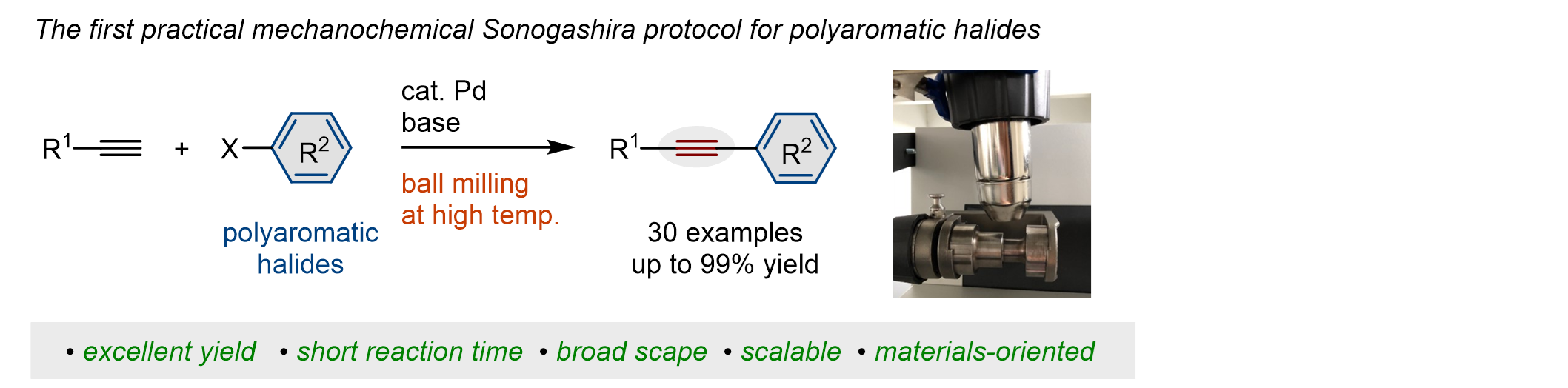
8.
Efficient Access to Materials-Oriented Aromatic Alkynes via the Mechanochemical Sonogashira Coupling of Solid Aryl Halides with Large Polycyclic Conjugated Systems
Gao, Y.; Feng, C.; Seo, T.; Kubota, K.*; Ito, H.* Chem. Sci. 2022, 13, 430–438.
DOI: 10.1039/D1SC05257H -
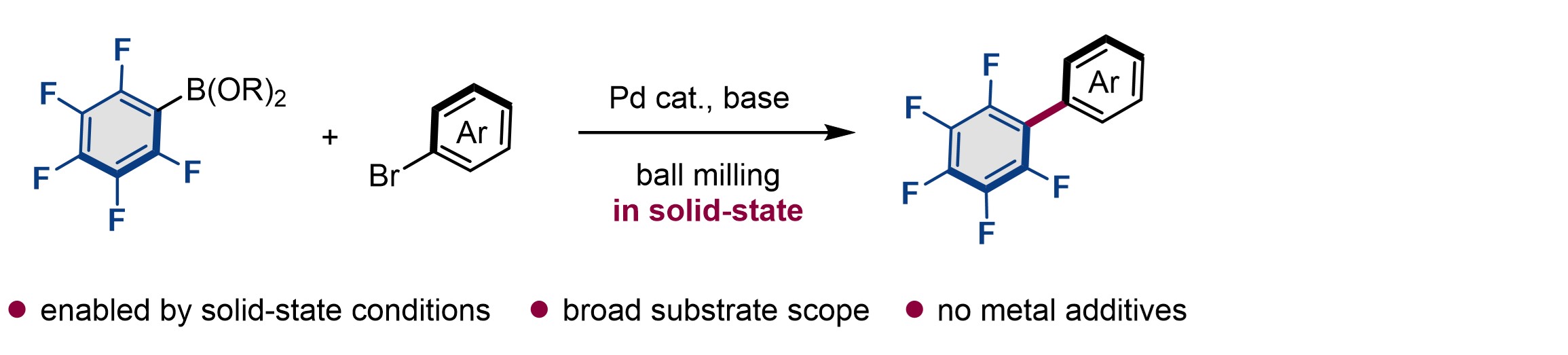
7.
Palladium-Catalyzed Solid-State Polyfluoroarylation of Aryl Halides Using Mechanochemistry
Takahashi, Rikuro; Seo, T.; Kubota, K.*; Ito, H.* ACS Catal. 2021, 11, 14803–14810.
DOI: 10.1021/acscatal.1c03731 -
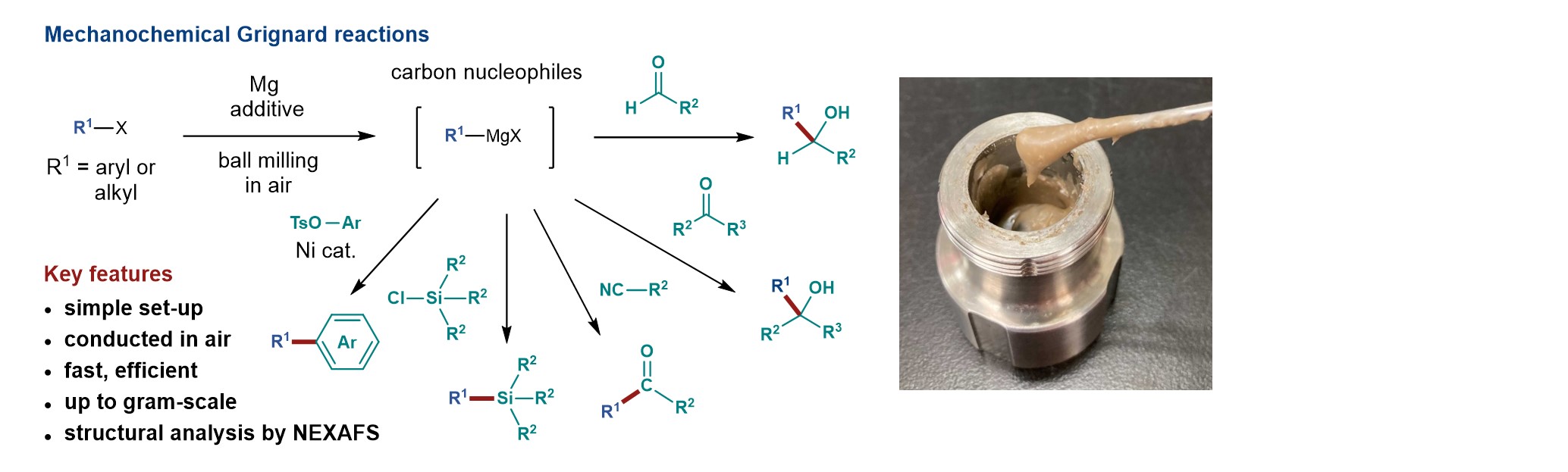
6.
Mechanochemical synthesis of magnesium-based carbon nucleophiles in air and their use in organic synthesis
Takahashi, R.; Hu, A.; Gao, P.; Gao, Y.; Pang, Y.; Seo, T.; Maeda, S.; Jiang, J.; Takaya, H.; Kubota, K.*; Ito, H.* Nature Commun. 2021, 12, 6691.
DOI: 10.1038/s41467-021-26962-w -
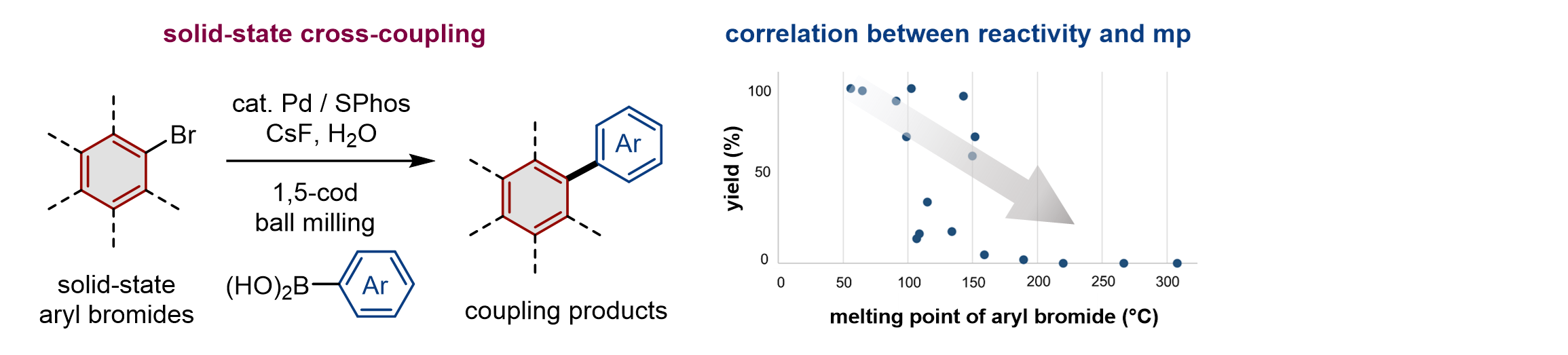
5.
Tackling Solubility Issues in Organic Synthesis: Solid-State Cross-Coupling of Insoluble Aryl Halides
Seo, T.; Toyoshima, N; Kubota, K.*; Ito, H.* J. Am. Chem. Soc. 2021, 143, 6165–6175.
DOI: 10.1021/jacs.1c00906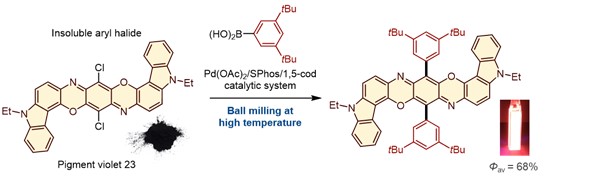 ・Highlighted in Synfacts: Solid-State Suzuki-Miyaura Cross-Coupling of Insoluble Aryl Halides Using a Ball-Milling Approach
・プレスリリース: 溶けない化合物でも使えるクロスカップリング反応の開発
・Highlighted in Synfacts: Solid-State Suzuki-Miyaura Cross-Coupling of Insoluble Aryl Halides Using a Ball-Milling Approach
・プレスリリース: 溶けない化合物でも使えるクロスカップリング反応の開発
-
4.
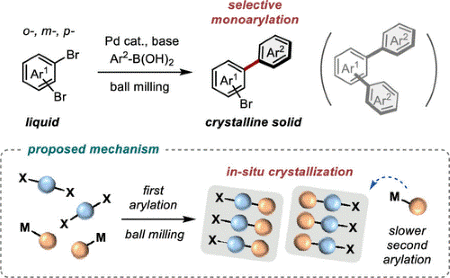
Selective Mechanochemical Monoarylation of Unbiased Dibromoarenes by In-situ Crystallization
Seo, T.; Kubota, K.*; Ito, H.* J. Am. Chem. Soc. 2020, 142, 9884–9889.
DOI: 10.1021/jacs.0c01739Under ball milling reaction conditions, palladium-catalyzed Suzuki–Miyaura cross-coupling reactions of unbiased dibromoarenes selectively afford the monoarylated products. This study gives a new approach that uses in-situ structure transitions in solids to achieve selective organic transformations difficult with traditional solution-based synthesis.
-
3.
Solid-State Suzuki-Miyaura Cross-Coupling Reactions: Olefin-Accelerated C–C Coupling Using Mechanochemistry
Seo, T.; Ishiyama, T.; Kubota, K.*; Ito, H.* Chem. Sci. 2019, 10, 8202–8210.
DOI: 10.1039/C9SC02185J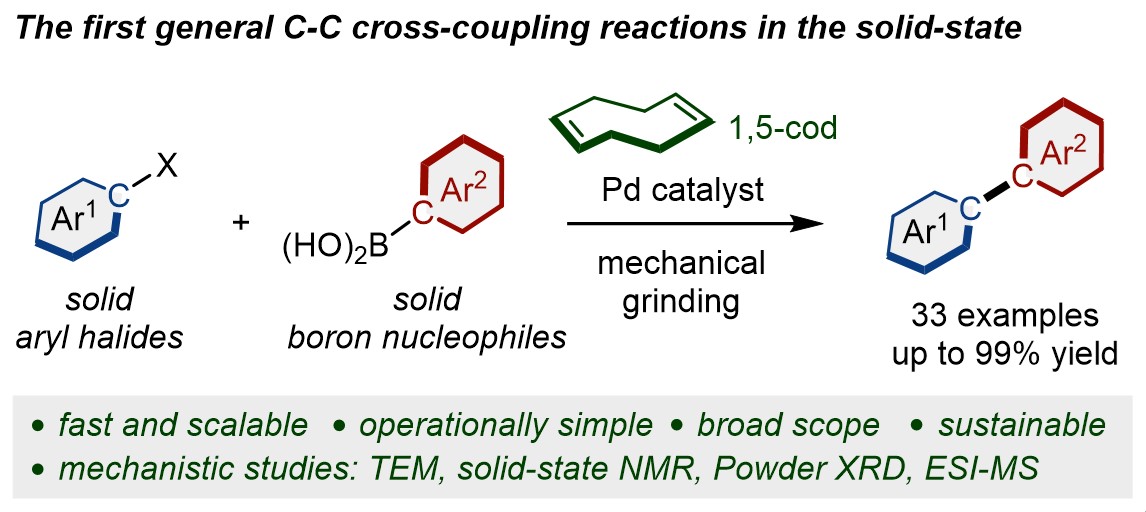 ・Highlighted in Chemistry World: Mechanochemistry bypasses need for solvents in Suzuki coupling
・Highlighted in Synfacts: Rebuilding from the Dust: Suzuki-Miyaura Cross-Coupling Using Mechanical Grinding
・Highlighted in Chemistry World: Mechanochemistry bypasses need for solvents in Suzuki coupling
・Highlighted in Synfacts: Rebuilding from the Dust: Suzuki-Miyaura Cross-Coupling Using Mechanical Grinding
-
2.
Olefin-accelerated solid-state C–N cross-coupling reactions using mechanochemistry
Kubota, K.*; Seo, T.; Koide, K.; Hasegawa, Y.; Ito, H.* Nature Communications 2019, 10, 111.
DOI: 10.1038/s41467-018-08017-9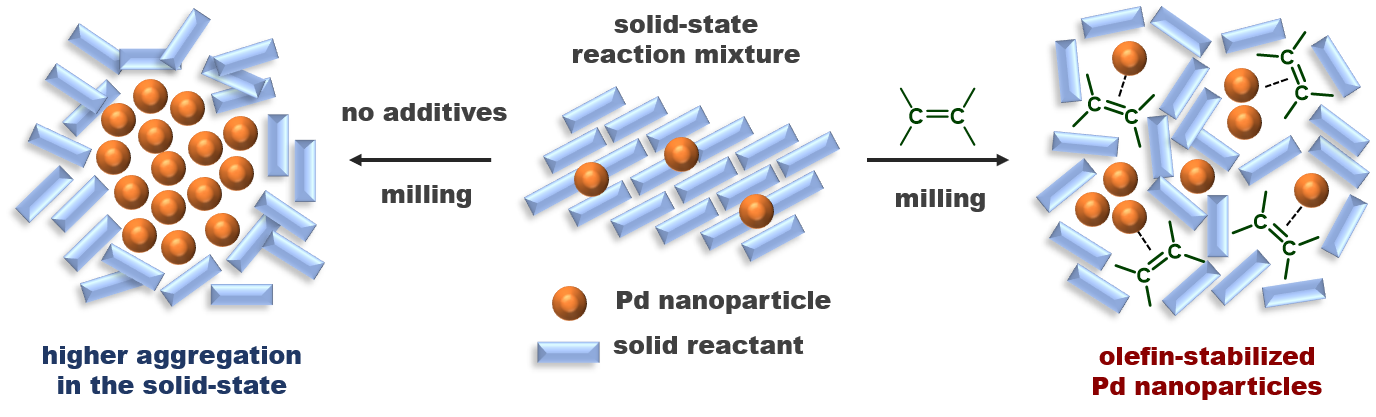 https://www.nature.com/articles/s41467-018-08017-9
・北海道大学 PRESS RELEASE 固体状態で進行するクロスカップリング反応を開発
・Behind the Paper (Nature Research Chemistry Community) Cross-Coupling in Solid Using Mechanochemistry
・Highlighted in News Paper: 日経産業新聞20190111
・Highlighted in News Paper: 日刊工業新聞20190115
・Highlighted in News Web: NHK 北大 医薬品合成で新手法開発
・Highlighted in News Web: 環境展望台 北大 固体状態で進行する有機合成反応プロセスを開発
・Selected as "Nature Communications Editor's Highlights"
・Highlighted in Phys.org.: Boosting solid state chemical reactions
・Highlighted in Converter News: Olefin Enables Efficient Solvent-Free Cross-Coupling Reactions
・Highlighted in Technology Networks: Olefin Makes Chemistry Greener
・Highlighted in Chem Station: スポットライトリサーチ ”メカノケミストリーを用いた固体クロスカップリング反応"
https://www.nature.com/articles/s41467-018-08017-9
・北海道大学 PRESS RELEASE 固体状態で進行するクロスカップリング反応を開発
・Behind the Paper (Nature Research Chemistry Community) Cross-Coupling in Solid Using Mechanochemistry
・Highlighted in News Paper: 日経産業新聞20190111
・Highlighted in News Paper: 日刊工業新聞20190115
・Highlighted in News Web: NHK 北大 医薬品合成で新手法開発
・Highlighted in News Web: 環境展望台 北大 固体状態で進行する有機合成反応プロセスを開発
・Selected as "Nature Communications Editor's Highlights"
・Highlighted in Phys.org.: Boosting solid state chemical reactions
・Highlighted in Converter News: Olefin Enables Efficient Solvent-Free Cross-Coupling Reactions
・Highlighted in Technology Networks: Olefin Makes Chemistry Greener
・Highlighted in Chem Station: スポットライトリサーチ ”メカノケミストリーを用いた固体クロスカップリング反応"
-
1.
Copper(I)-Catalyzed Stereoselective Defluoroborylation of Aliphatic Gem-Difluoroalkenes
Ito, H.*; Seo, T.; Kojima, R.; Kubota, K. Chem. Lett. 2018, 47, 1330–1332.
DOI: 10.1246/cl.180656