RESEARCH
INTRODUCTION
Our research group aims to develop new and useful synthetic reactions, functional materials, and catalysts by combining carbon-based molecules with other elements (silicon, boron, phosphorus, typical elements, and transition metals). We hope to create new research fields by introducing new concepts in organic chemistry.
In addition, by having our students conduct high-level research, we aim to educate and train pioneering researchers who will work at the forefront of organic chemistry fields in the future.
We are also expanding our research through joint research with several companies, domestic universities, and international universities.
RESEARCH TOPICS
-
RESEARCH
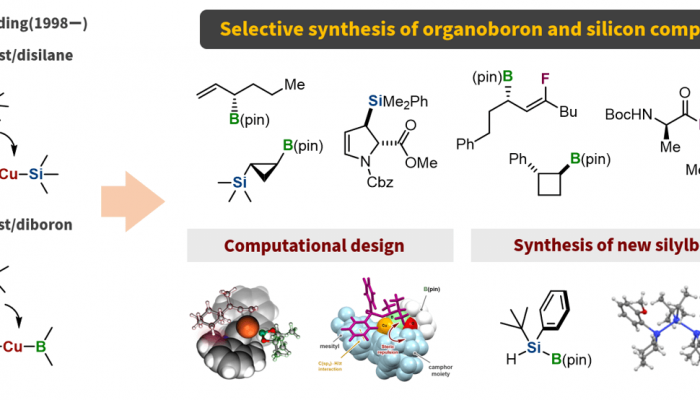
Development of selective synthesis of organoboron and organosilicon compounds
Organoboron and organosilicon compounds are widely used in synthetic organic chemistry. These organometallic compounds are highly stable; however, they exhibit diverse reactivities when activated by appropriate reagents or transition-metal catalysts. It has also been demonstrated that these organic compounds are useful pharmaceuticals and functional organic materials. However, the synthesis of highly functionalized organoboron and organosilicon compounds remains difficult, and the development of new selective synthetic methods is required. In 1998, we discovered a nucleophilic silylation reaction using a copper(I) catalyst with a disilane compound as the silylation reagent. Furthermore, based on the similar reactivity of silicon and boron compounds toward transition metals, we investigated copper(I)-catalyzed reactions using a diboron compound as a borylation reagent and observed that nucleophilic boryl addition reaction to conjugated carbonyl compounds proceeded. These discoveries have led to the development of several new synthetic methods for organoboron and silicon compounds. More recently, we succeeded in the rational design of chiral copper(I) catalysts using quantum chemical calculations and in the development of general synthetic methods for silylborane compounds that can act as silicon nucleophiles under basic conditions. We will continue this research to push the boundaries of organoboron and organosilicon chemistry.
-
RESEARCH
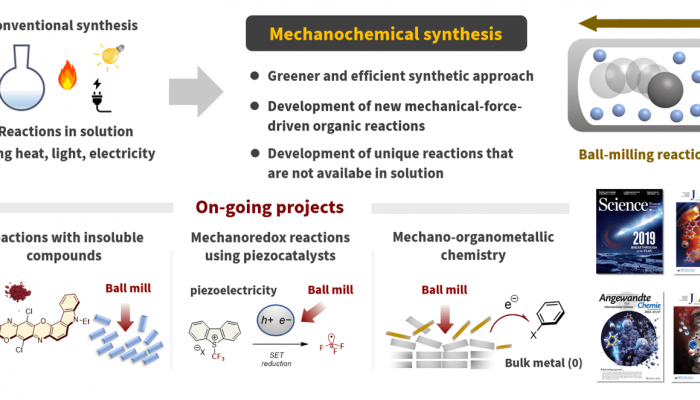
Development of mechanochemical organic reactions
To date, several useful organic synthetic reactions have been developed, most of which are performed in solutions using organic solvents. Therefore, pharmaceutical and fine chemicals industries significantly depend on solvent-based organic synthesis, which has resulted in serious problems with regard to solvent waste, as organic solvents usually account for approximately 80–90% of the total mass used in any organic reaction. In this context, mechanochemical synthesis using ball milling without organic solvents has garnered significant attention as a greener way to perform organic reactions. In mechanochemical synthesis, strong mechanical agitation by ball milling enables solvent-free reactions with high efficiencies. However, its application in organic synthetic reactions has been limited, and its synthetic potential remains unclear. We began our mechanochemical organic synthesis research in 2018, focusing on the development of “reactions only possible in a ball mill”. We have succeeded in developing selective organic synthetic reactions that are only feasible in the solid state, molecular transformations of insoluble compounds, and new mechanical-force-driven organic reactions that have rarely been developed by conventional synthetic organic chemists. This research will contribute not only to the development of basic science but also to the creation of a new environmentally benign synthetic method to provide medicines, bioactive substances, and organic molecular materials to human societies in an environmentally friendly manner. We will continue to explore the potential of this mechanochemical organic synthesis.
-

RESEARCH
Development of functional molecular crystalline materials based on solid-state molecular dynamics
Generally, molecules in the solid state are tightly packed; therefore, it is difficult to show movement in the solid state. However, some molecular crystals can alter the molecular arrangement in crystalline media when external stimuli (energy), such as heat, light, and force, are applied to the crystalline sample, which can induce color or emission changes. Several physical properties of crystals are highly intercorrelated with their nanoscale crystal structures. Thus, the molecular arrangement transition in a crystal by an external stimulus can interplay as one of the fundamental motifs in the design and control of the solid-state functional properties. However, the rational design of these molecular crystals is still challenging because of the difficulty in predicting the molecular packing in crystals. In 2008, we discovered the mechanochromic properties of molecular crystals comprising an aryl Au(I) isocyanide complex, which exhibited a drastic emission color change from blue to yellow upon mechanical grinding. In addition, the changed emission color could be converted to the initial emission by simply adding an organic solvent. Further studies have demonstrated that the color alteration originates from the change in molecular arrangement via mechanical stress or the addition of organic solvents. Upon conducting a more in-depth analysis, we observed that even partial mechanical pricking can induce a single-crystal-to-single-crystal (SCSC) phase transition with emission alteration. Notably, the propagation of the molecular arrangement change in a single crystal can be visualized by the corresponding emission alteration, as shown in the figure below (we defined this as “molecular domino”). The emission properties were determined by the formation or cleavage of aurophilic interactions between the Au(I) complexes in the crystal. The observed SCSC transitions were not reversible. However, recently, we discovered that some Au(I) isocyanide complexes possessing large dipoles can produce emission crystals exhibiting reversible SCSC transitions with drastic emission color changes switched by mechanical stress and MeOH vapor. In addition to the molecular arrangement alternation in the crystal, we also utilized molecular rotation to control the solid-state emission. The luminescence properties of molecular crystals significantly correlate with the luminophore arrangement and structural conformations in the solid state. In other words, utilizing molecular rotation in the solid state could be one of the motifs to design stimuli-responsive solid-state luminescence. Recently, we reported the first example of solid-state phosphorescence controlled by molecular rotation in the crystalline phase using dumbbell-shaped Au(I) complexes, as shown in the figure below. Upon cooling the crystal, the rotational motion became static and the emission color changed to yellow. In addition, we developed a novel platform for designing molecular rotations in the solid state in a rational way by utilizing the steric and electronic effects of NHC metal complexes. This structural motif provides guidance for controlling molecular rotation and the associated emission properties.
-

RESEARCH
Collaborative research (companies, domestic universities, and international universities)
Mechanochemical organic synthesis, which has been studied since 2018, offers several advantages that are not present in conventional solution-based organic synthesis, such as the significant reduction of reaction solvents, faster reaction kinetics, reactions with insoluble substrates, and activation of solid substrates by mechanical forces. To further advance research in these areas, we are promoting collaborative efforts between companies, domestic universities, and international universities.
Contactus
- Professor Dr. Hajime Ito
-
E-mail: hajitoeng.hokudai.ac.jp
