Generally, molecules in the solid state are tightly packed; therefore, it is difficult to show movement in the solid state. However, some molecular crystals can alter the molecular arrangement in crystalline media when external stimuli (energy), such as heat, light, and force, are applied to the crystalline sample, which can induce color or emission changes. Several physical properties of crystals are highly intercorrelated with their nanoscale crystal structures. Thus, the molecular arrangement transition in a crystal by an external stimulus can interplay as one of the fundamental motifs in the design and control of the solid-state functional properties. However, the rational design of these molecular crystals is still challenging because of the difficulty in predicting the molecular packing in crystals.
In 2008, we discovered the mechanochromic properties of molecular crystals comprising an aryl Au(I) isocyanide complex, which exhibited a drastic emission color change from blue to yellow upon mechanical grinding. In addition, the changed emission color could be converted to the initial emission by simply adding an organic solvent. Further studies have demonstrated that the color alteration originates from the change in molecular arrangement via mechanical stress or the addition of organic solvents.

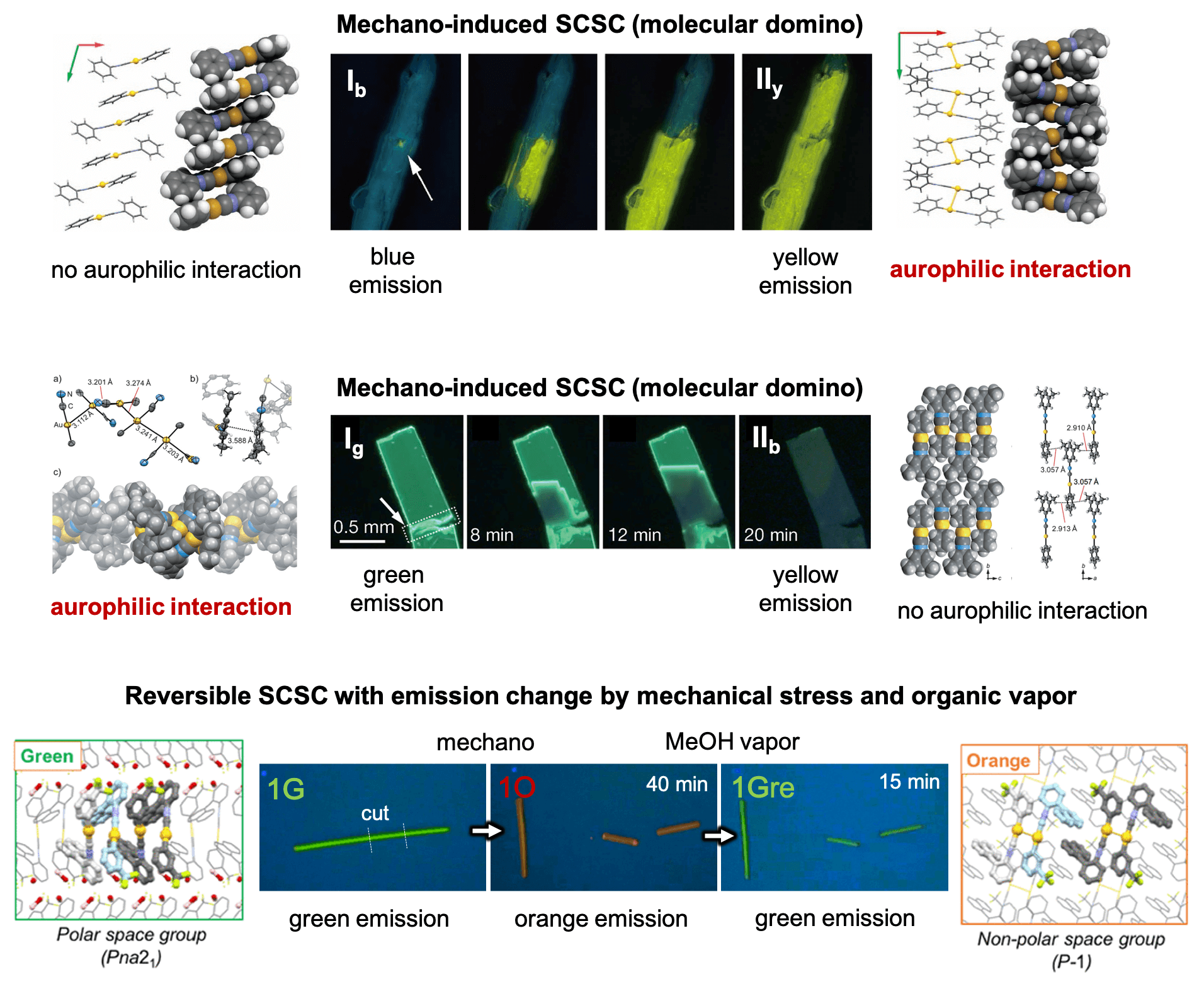
Upon conducting a more in-depth analysis, we observed that even partial mechanical pricking can induce a single-crystal-to-single-crystal (SCSC) phase transition with emission alteration. Notably, the propagation of the molecular arrangement change in a single crystal can be visualized by the corresponding emission alteration, as shown in the figure below (we defined this as “molecular domino”). The emission properties were determined by the formation or cleavage of aurophilic interactions between the Au(I) complexes in the crystal. The observed SCSC transitions were not reversible. However, recently, we discovered that some Au(I) isocyanide complexes possessing large dipoles can produce emission crystals exhibiting reversible SCSC transitions with drastic emission color changes switched by mechanical stress and MeOH vapor.
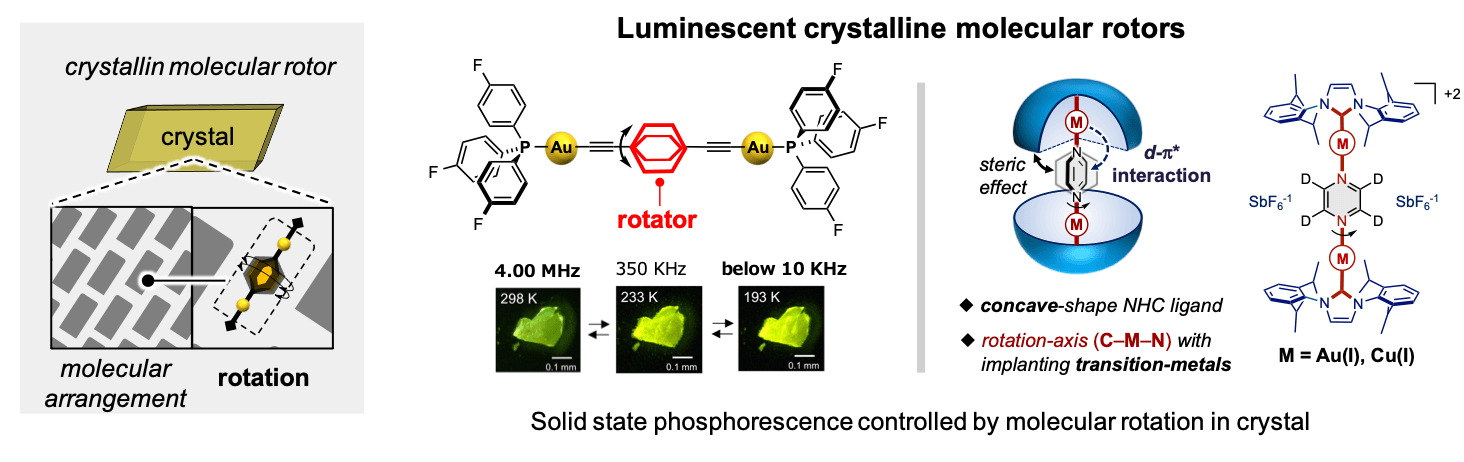
In addition to the molecular arrangement alternation in the crystal, we also utilized molecular rotation to control the solid-state emission. The luminescence properties of molecular crystals significantly correlate with the luminophore arrangement and structural conformations in the solid state. In other words, utilizing molecular rotation in the solid state could be one of the motifs to design stimuli-responsive solid-state luminescence. Recently, we reported the first example of solid-state phosphorescence controlled by molecular rotation in the crystalline phase using dumbbell-shaped Au(I) complexes, as shown in the figure below. Upon cooling the crystal, the rotational motion became static and the emission color changed to yellow. In addition, we developed a novel platform for designing molecular rotations in the solid state in a rational way by utilizing the steric and electronic effects of NHC metal complexes. This structural motif provides guidance for controlling molecular rotation and the associated emission properties.
SELECTED PUBLICATIONS
Luminescent mechanochromism
- Reversible Mechanochromic Luminescence of [(C6F5Au)2(μ-1,4-diisocyanobenzene)]Ito, H.*; Saito, T.; Oshima, N.; Kitamura, N.; Ishizaka, S.; Hinatsu, Y.; Wakeshima, M.; Kato, M.; Tsuge, K.; Sawamura, M.* J. Am. Chem. Soc. 2008, 130, 10044.
DOI: 10.1021/ja8019356
- Mechano-Responsive Luminescence via Crystal-to-Crystal Phase Transitions between Chiral and Non-Chiral Space GroupsJin, M.; Seki, T.*; Ito, H.* J. Am. Chem. Soc. 2017, 139, 7452 – 7455.
DOI: 10.1021/jacs.7b04073
- Luminescent Mechanochromic 9-Anthryl Gold(I) Isocyanide Complex with an Emission Maximum at 900 nm after Mechanical StimulationSeki, T.*; Tokodai, N.; Omagari, S.; Nakanishi, T. ; Hasegawa, Y.; Iwasa, T.; Taketsugu, T.; Ito, H.* J. Am. Chem. Soc. 2017, 139, 6514 – 6517.
DOI: 10.1021/jacs.7b00587
- A Screening Approach for the Discovery of Mechanochromic Gold(I) Isocyanide Complexes with Crystal-to-Crystal Phase TransitionsSeki, T.*; Takamatsu, Y; Ito, H.* J. Am. Chem. Soc. 2016, 138, 6252 – 6260.
DOI: 10.1021/jacs.6b02409
Single-crystal-to-single-crystal (SCSC) Phase transition as molecular domino
- Mechanical stimulation and solid seeding trigger single-crystal-to-single-crystal molecular
domino transformationsIto, H.;* Muromoto, M.; Kurenuma, S.; Ishizaka, S.; Kitamura, N.; Sato, H.; Seki, T. Nature Commun. 2019, 4, 2009.
DOI: 10.1038/ncomms3009
- Controlling Mechano- and Seeding-Triggered Single-Crystal-to-Single-Crystal Phase Transition: Molecular Domino with a Disconnection of Aurophilic BondsSeki, T.; Sakurada, K.; Ito, H.* Angew. Chem., Int. Ed. 2013, 52, 12828-12832. VIP paper
DOI: 10.1002/anie.201307672
- Mechanical-Stimulation-Triggered and Solvent-Vapor-Induced Reverse Single-Crystal-to-Single-Crystal Phase Transitions with Alterations of the Luminescence ColorJin, M.; Sumitani, T.; Sato, H.; Seki, T.*; Ito, H.* J. Am. Chem. Soc. 2018, 140, 2875 – 2879.
DOI: 10.1021/jacs.7b12455
Dancing crystals
- Mechanical deformation and multiple thermal restoration of organic crystals: reversible multi-stage shape-changing effect with luminescence-color changesFeng, C.; Seki, T.*; Sakamoto, S.; Sasaki, T.; Takamizawa, S.*; Ito, H.* Chem. Sci. 2022, Edge Article
DOI: 10.1039/D2SC03414J
Crystal jumping
- Photoinduced Single-Crystal-to-Single-Crystal Phase Transition and Photosalient Effect of a Gold(I) Isocyanide Complex with Shortening Intermolecular Aurophilic BondsSeki, T.; Sakurada, K.; Muromoto, M.; Ito, H.* Chem. Sci. 2015, 6, 1491 – 1497. Open Access Article.
DOI: 10.1039/C4SC02676D
- Anisotropic strain release in a thermosalient crystal: Correlation between the microscopic orientation of molecular rearrangements and the macroscopic mechanical motionSeki, T.*; Mashimo, T.; Ito, H.* Chem. Sci. 2019, 10, 4185 – 4191.
DOI: 10.1039/C8SC05563G
Luminescent crystalline molecular rotors
- Encapsulating N-Heterocyclic Carbene Binuclear Transition-Metal Complexes as a New Platform for Molecular Rotation in Crystalline Solid-StateJin, M.*; Ando, R.; Jellen, M. J.; Garcia-Garibay, M. A.; Ito, H.* J. Am. Chem. Soc. 2021, 143, 1144 – 1153.
DOI: https://doi.org/10.1021/jacs.0c11981
- Anisotropic Thermal Expansion as the Source of Macroscopic and Molecular Scale Motion in Phosphorescent Amphidynamic CrystalsJin, M.; Yamamoto, S.; Seki, T.; Ito, H.*; Garcia-Garibay, M. A.* Angew. Chem. Int. Ed. 2019, 58, 18003 – 18010.
DOI: 10.1002/anie.201909048
- Phosphorescence Control Mediated by Molecular Rotation and Aurophilic Interactions in Amphidynamic Crystals of 1,4-bis[tri-(p-fluorophenyl)phosphane-gold(I)-ethynyl]benzeneJin, M.; Chung, T. J.; Seki, T.; Ito, H.*; Garcia-Garibay, M. A.* J. Am. Chem. Soc. 2017, 139, 18115–18121.
DOI: 10.1021/jacs.7b11316



