Development of mechanochemical organic reactions
To date, several useful organic synthetic reactions have been developed, most of which are performed in solutions using organic solvents. Therefore, pharmaceutical and fine chemicals industries significantly depend on solvent-based organic synthesis, which has resulted in serious problems with regard to solvent waste, as organic solvents usually account for approximately 80–90% of the total mass used in any organic reaction. In this context, mechanochemical synthesis using ball milling without organic solvents has garnered significant attention as a greener way to perform organic reactions. In mechanochemical synthesis, strong mechanical agitation by ball milling enables solvent-free reactions with high efficiencies. However, its application in organic synthetic reactions has been limited, and its synthetic potential remains unclear.
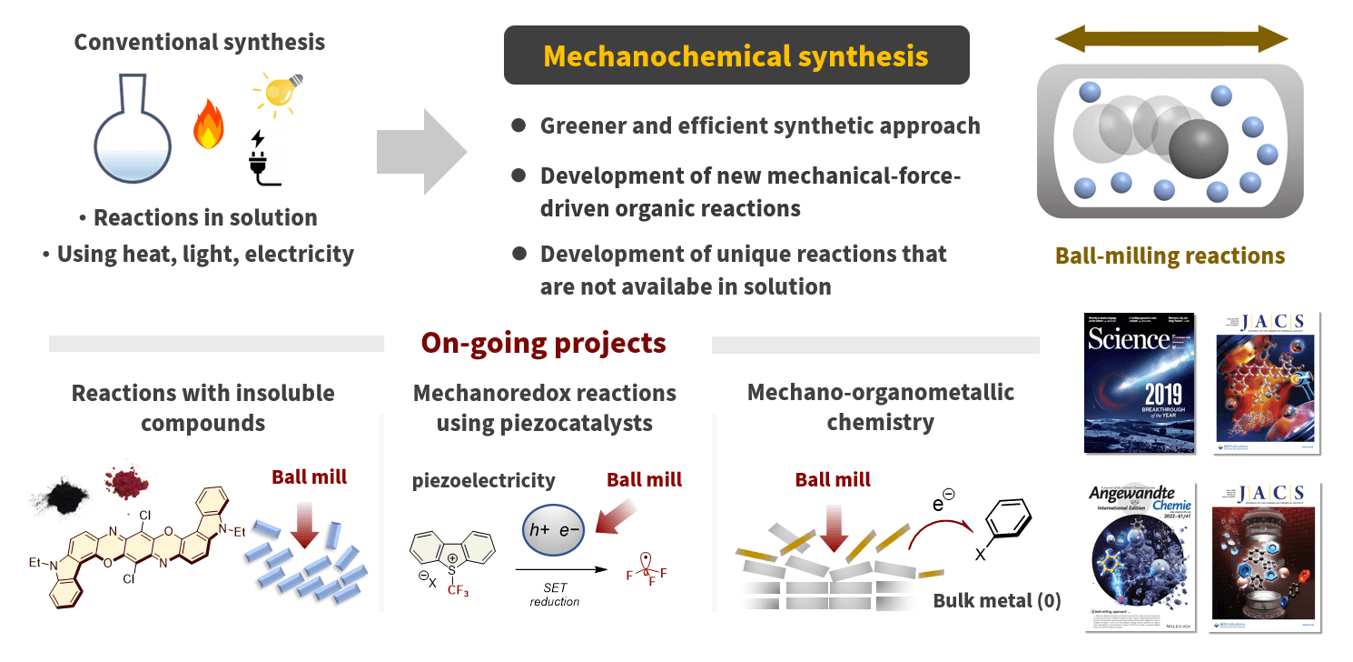
We began our mechanochemical organic synthesis research in 2018, focusing on the development of “reactions only possible in a ball mill”. We have succeeded in developing selective organic synthetic reactions that are only feasible in the solid state, molecular transformations of insoluble compounds, and new mechanical-force-driven organic reactions that have rarely been developed by conventional synthetic organic chemists. This research will contribute not only to the development of basic science but also to the creation of a new environmentally benign synthetic method to provide medicines, bioactive substances, and organic molecular materials to human societies in an environmentally friendly manner. We will continue to explore the potential of this mechanochemical organic synthesis.
