Organoboron and organosilicon compounds are widely used in synthetic organic chemistry. These organometallic compounds are highly stable; however, they exhibit diverse reactivities when activated by appropriate reagents or transition-metal catalysts. It has also been demonstrated that these organic compounds are useful pharmaceuticals and functional organic materials. However, the synthesis of highly functionalized organoboron and organosilicon compounds remains difficult, and the development of new selective synthetic methods is required.
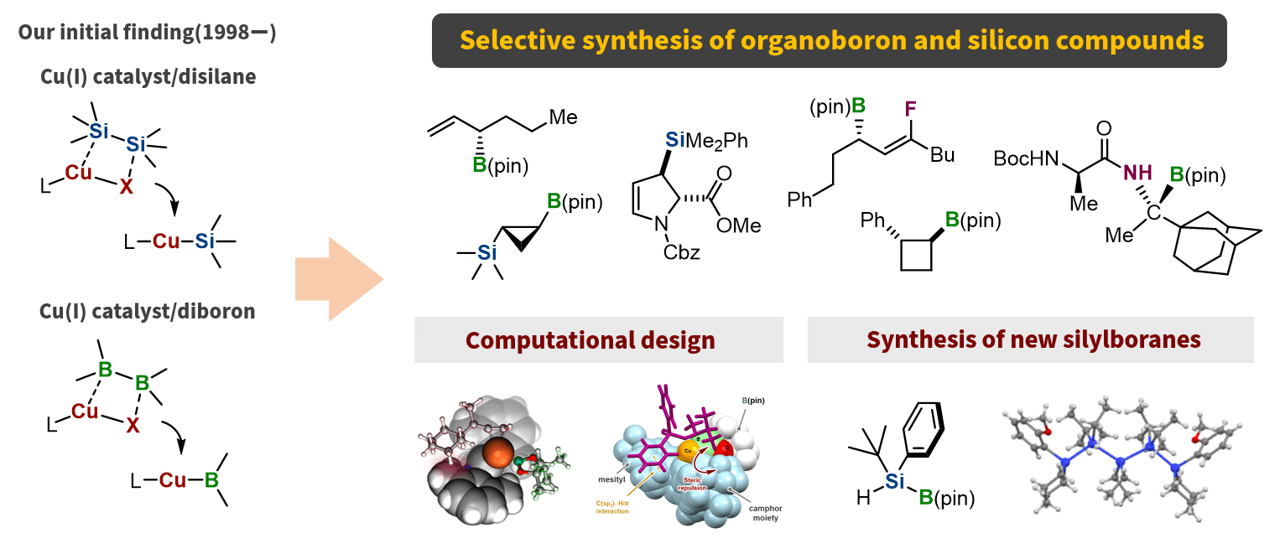
In 1998, we discovered a nucleophilic silylation reaction using a copper(I) catalyst with a disilane compound as the silylation reagent. Furthermore, based on the similar reactivity of silicon and boron compounds toward transition metals, we investigated copper(I)-catalyzed reactions using a diboron compound as a borylation reagent and observed that nucleophilic boryl addition reaction to conjugated carbonyl compounds proceeded. These discoveries have led to the development of several new synthetic methods for organoboron and silicon compounds. More recently, we succeeded in the rational design of chiral copper(I) catalysts using quantum chemical calculations and in the development of general synthetic methods for silylborane compounds that can act as silicon nucleophiles under basic conditions. We will continue this research to push the boundaries of organoboron and organosilicon chemistry.
SELECTED PUBLICATIONS
- Conformationally Fixed Chiral Bisphosphine Ligands by Steric Modulators on the Ligand Backbone: Selective Synthesis of Strained 1,2-Disubstituted Chiral cis-Cyclopropanes.
Iwamoto, H.; Ozawa, Y.; Hayashi, Y.; Imamoto, T.; Ito, H.* J. Am. Chem. Soc. 2022, 144, 10483–10494.
DOI: 10.1021/jacs.2c02745
- Regio- and Stereoselective Synthesis of Multi-Alkylated Allylic Boronates through Three-Component Coupling Reactions between Allenes, Alkyl Halides, and a Diboron Reagent.
Ozawa, Y.; Endo, K.; Ito, H.* J. Am. Chem. Soc. 2021, 143, 13865–13877.
DOI: 10.1021/jacs.1c06538
- Synthesis of Hydrosilylboronates via the Monoborylation of a Dihydrosilane Si−H Bond and Their Application for the Generation of Dialkylhydrosilyl Anions.
Takeuchi, T.; Shishido, R.; Kubota, K.*; Ito, H.* Chem. Sci. 2021, 12, 11799–11804.
DOI: 10.1039/D1SC01440D
- A Copper(I)-Catalyzed Radical Relay Reaction Enabling the Intermolecular 1,2-Alkylborylation of Unactivated Olefins
Akiyama, S.; Oyama, N.; Endo, T.; Kubota, K.; Ito, H.* J. Am. Chem. Soc. 2021, 143, 5260–5268.
DOI: 10.1021/jacs.1c02050
- General Synthesis of Trialkyl- and Dialkylarylsilylboranes: Versatile Silicon Nucleophiles in Organic Synthesis
Shishido, R.; Uesugi, M.; Takahashi, Rikuro.; Mita, T.; Ishiyama, T.; Kubota, K.*; Ito, H.* J. Am. Chem. Soc. 2020, 142, 14125–14133.
DOI: 10.1021/jacs.0c03011
- Computational Design of High-performance Ligand for Enantioselective Markovnikov Hydroboration of Aliphatic Terminal Alkenes.
Iwamoto, H.; Imamoto, T.; Ito, H.* Nature Commun. 2018, 9, 2290.
DOI: 10.1038/s41467-018-04693-9
- Anomalous Reactivity of Silylborane: Transition-Metal-Free Boryl Substitution of Aryl, Alkenyl, and Alkyl Halides with Silylborane/Alkoxy Base Systems
Yamamoto, E.; Izumi, K.; Horita,Y.; Ito, H. J. Am. Chem. Soc. 2012, 134, 19997–20000.
DOI: 10.1021/ja309578k
- Copper-catalyzed γ-Selective and Stereospecific Substitution Reaction of Allylic Carbonates with Diboron: Efficient Route to Chiral Allylboron Compounds
Ito, H.; Kawakami, C.; Sawamura, M. J. Am. Chem. Soc. 2005, 127, 16034–16035.
DOI: 10.1021/ja056099x
- Boration of an α,β-Enone Using a Diboron Promoted by a Copper(I)-Phosphine Mixture Catalyst.
Ito, H.; Yamanaka, H.; Tateiwa, J.-i.; Hosomi, A. Tetrahedron Lett. 2000, 41, 6821–6825.
DOI: 10.1016/S0040-4039(00)01161-8
- New Method for Introduction of a Silyl Group into α,β-Enones using a Disilane Catalyzed by a Copper(I) Salt.
Ito, H.; Ishizuka, T.; Tateiwa, J.-i.; Sonoda, M.; Hosomi, A. J. Am. Chem. Soc. 1998, 120, 11196–11197.
DOI: 10.1021/ja9822557

